The Food and Drug Administration on Monday granted full approval of the Pfizer COVID-19 vaccine, becoming the first covid-19 vaccine to transition from an emergency authorization status to full FDA approval.
The approval comes in a week prior to federal health officials’ earlier estimates that the agency would complete its review by Labor Day.
The full approval indicates that Pfizer has shown enough effectiveness and safety data to meet the stringent Biologics License Application (BLA) requirements, which includes at least six months of safety data from a majority of the volunteers in a large, final stage clinical trial.
“Based on the longer-term follow-up data that we submitted, today’s approval for those aged 16 and over affirms the efficacy and safety profile of our vaccine at a time when it is urgently needed,” Albert Bourla, Pfizer CEO said in a statement to ABC. “Hundreds of millions of doses of our vaccine already have been administered in the U.S. since December 2020, and we look forward to continuing to work with the U.S. government to reach more Americans.”
MORE: 5 good reasons for the FDA to give full approval to COVID-19 vaccines: Analysis
This prioritized review entailed government scientists pouring over hundreds of thousands of pages of safety and efficacy data at a rapid-fire pace, conducting meticulous inspections of Pfizer’s manufacturing process.


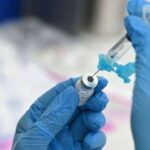
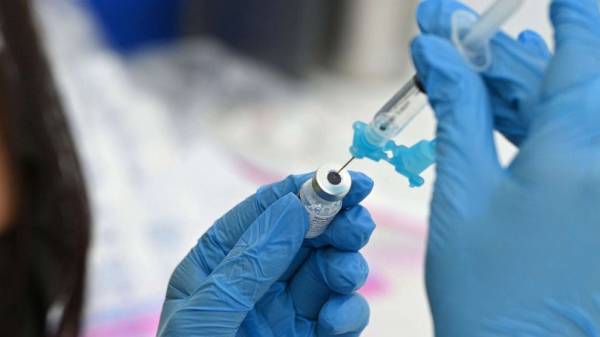
Robyn Beck/AFP via Getty Images, FILEA healthcare worker fills a syringe with Pfizer COVID-19 vaccine at a community vaccination event in Los Angeles, Aug. 11, 2021.
Pfizer’s full and formal approval will now pave the way for further vaccine mandates in both the public and private sector, akin to existing vaccine mandates for other FDA-approved vaccines. Some businesses and state leaders have held off thus far, signaling they’d wait for full approval before imposing tighter requirements.
Federal, state and local health officials have also expressed optimism that full approval will help dissolve some of the lingering hesitancy around taking a shot that until now has been only authorized for emergency use — a forecast recent polling has also reflected.
MORE: COVID-19 live updates: Rev. Jesse Jackson 'responding' to hospital treatment
U.S. Surgeon General Dr. Vivek Murthy, predicting on ABC’s “This Week” Sunday that full approval may “tip” some fence-sitters towards taking the shot, and prompt more workplaces and schools to move forward on requirements.
“I am hopeful this approval will help increase confidence in our vaccine, as vaccination remains the best tool we have to help protect lives and achieve herd immunity,” Bourla said upon Pfizer’s approval.
“Full approval could not come at a more important time, as the highly contagious Delta variant continues to drive up caseloads and deaths across the U.S.,” Dr. Rich Besser, former acting CDC director and president of the Robert Wood Johnson Foundation said. “I am hopeful that full approval will address any remaining concerns and will move many people to a ‘yes’ on vaccination.”
MORE: FDA says its 'working as quickly as possible' to review for full approval of vaccines
Pfizer was the first to request full approval in the U.S.; other Covid vaccine makers are likely to follow suit. All three authorized vaccines were granted emergency authorization based on massive clinical trials involving tens of thousands of volunteers.
Federal health officials have come under immense political pressure from all sides to get the approval done as soon as possible — as much of the country faces yet another surge, and the Delta variant threatens hard-fought wins in the fight against the virus.
In private calls with the White House Covid team obtained by ABC News, some of nation’s governors have recently expressed waning patience and frustration over the wait for full approval of the vaccine — saying the FDA either needs to act, or be transparent about how much longer there is to wait — given that the lack of full approval is a recurring reason they had heard among the hesitant for not taking the shot yet.
The FDA had made clear getting Pfizer’s vaccine to the approval finish line is a top priority, with an “all hands on deck approach” and “moving forward as rapidly as possible.”
ABC News learned in late July the agency would reprioritize some of its personnel and technology resources from “across the agency” and reshuffling other existing work, in order to finish the review faster, devoting those additional resources towards expediting the process for Pfizer’s approval.
“We recognize that for some, the FDA approval of COVID-19 vaccines may bring additional confidence and encourage them to get vaccinated,” an agency spokesperson told ABC in late July, promising any approved vaccine would meet “rigorous standards for safety, effectiveness, and quality.”
Comments (0)Share to FacebookShare to TwitterEmail this article

Sourse: abcnews.go.com